California Signs PBM Law: As Federal PBM Regulation Lags, the States Step Up
With federal PBM reform stalled, states like California are stepping up with laws like SB 41 to boost transparency and lower drug costs.
With federal PBM reform stalled, states like California are stepping up with laws like SB 41 to boost transparency and lower drug costs.
California’s leading facial plastic surgeons redefine revision facelifts through precision, innovation and patient-centered artistry. Find out which ones get the best results.
Profound opportunities and challenges are playing out at the intersection of the two.

A much diminished 23andMe exists today, a far cry from the phenomenon that it was in the world consumer DNA testing. Could things have unfolded differently under a different leader with a different vision?

Carmot Therapeutics isn’t the first company targeting both the GLP-1 and GIP receptors to treat metabolic diseases. But the company contends that biased signaling—activating certain pathways but not others—is key to setting its drugs apart from others in the same class.

In a landscape where complexity has long been the norm, the power of one lies not just in unification, but in intelligence and automation.

Bayer opens its new cell therapy manufacturing facility as it prepares a Parkinson’s disease cell therapy for Phase 2 testing. The new 100,000 square foot facility also has space for manufacturing other cell therapies in the pharma giant’s pipeline.

Avalon BioVentures unveiled new portfolio company Arialys Therapeutics. The startup’s lead program, an antibody from Astellas Pharma, is a potential treatment for anti-NMDA receptor encephalitis (ANRE), a rare autoimmune inflammatory disorder.

Amgen and the Federal Trade Commission are settling the lawsuit the regulator filed to block the pharmaceutical giant’s $28 billion Horizon Therapeutics acquisition. As part of the settlement, Amgen agrees not to “bundle” its products with Horizon’s drugs in negotiations with health plans.

Santa Clara Valley Healthcare in California has begun notifying 43,000 patients about their eligibility for billing corrections and refunds. The system’s patient outreach effort is a result of a recently settled lawsuit filed against Santa Clara County. In the complaint, former patients alleged the county did not inform them about its hospitals' charity care and discount payment policies, leading them to have to pay large bills.
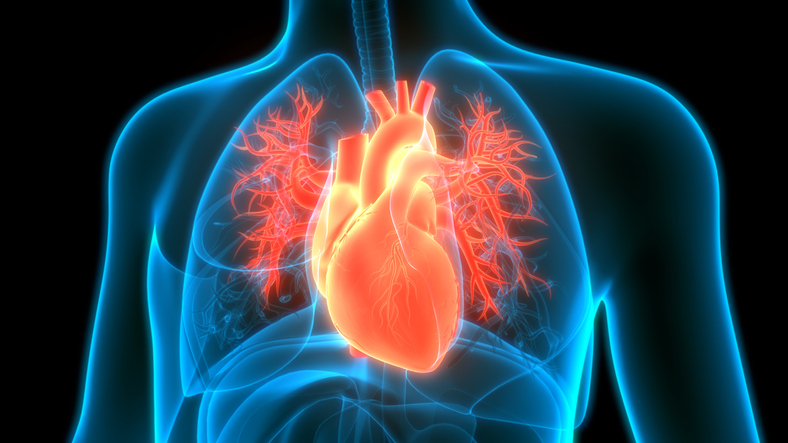
Novartis and Ionis Pharmaceuticals, already partners in cardiovascular disease R&D, are expanding their alliance to see if they can develop a next-generation therapy offering efficacy and dosing advantages. But competitors are already in the mix with programs addressing the same cardiovascular disease target.

Octave Bioscience, based in Menlo Park, California, just closed a $30 million extension to its Series B funding round.
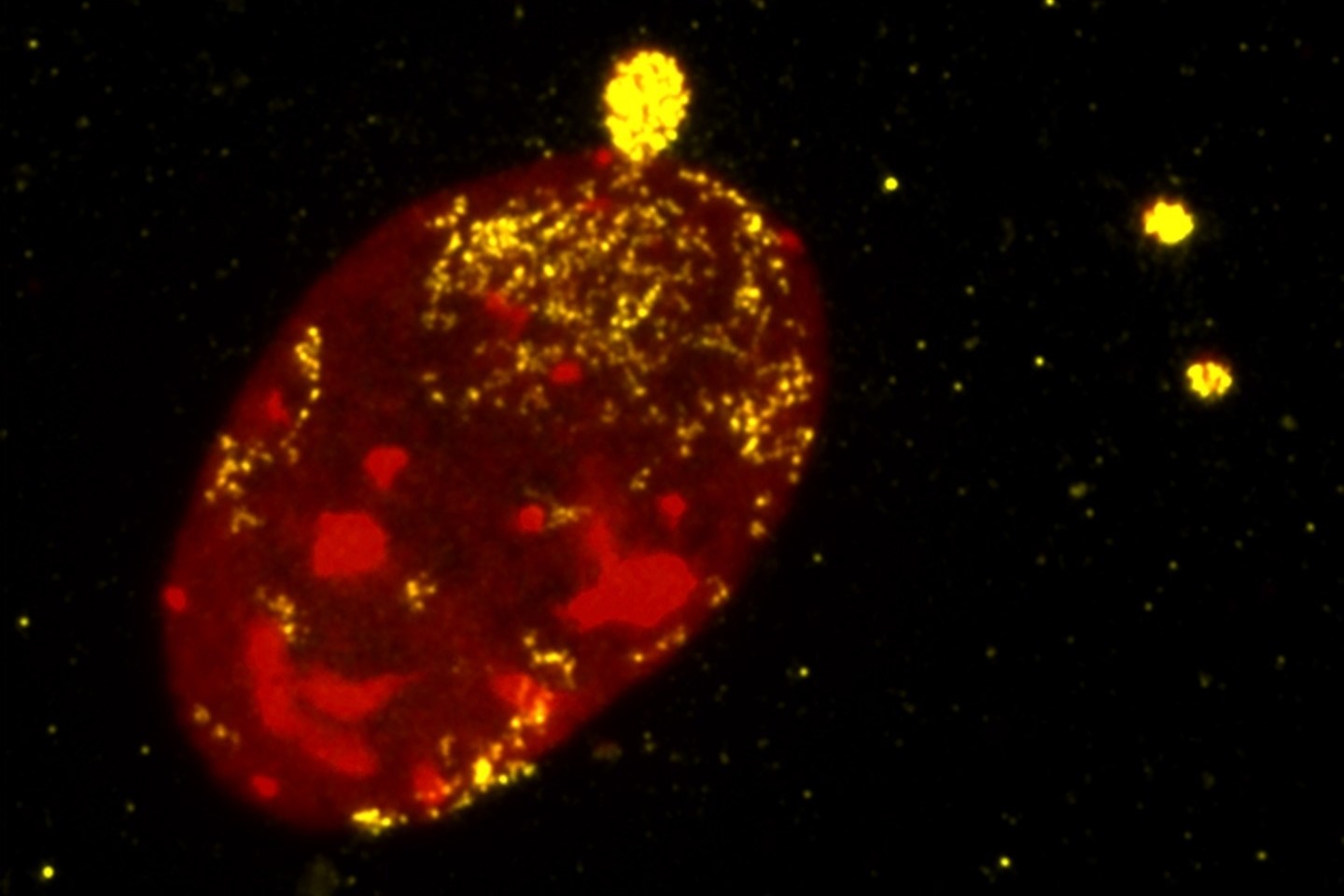
Turnstone Biologics isn’t the only cell therapy biotech focused on tumor-infiltrating lymphocytes. With $80 million in IPO cash, it can continue clinical trials that could show whether its approach to TILs has advantages.

The FDA approved Roctavian for treating hemophilia A. The regulatory decision makes the BioMarin Pharmaceutical product the first gene therapy for this inherited bleeding disorder.

Healthcare dealmakers must prepare for a new law and a recently-introduced bill in the California Senate, both of which aim to increase oversight in the healthcare M&A space. This is a legislative trend other states are beginning to participate in too — such as New York, Massachusetts and Connecticut.